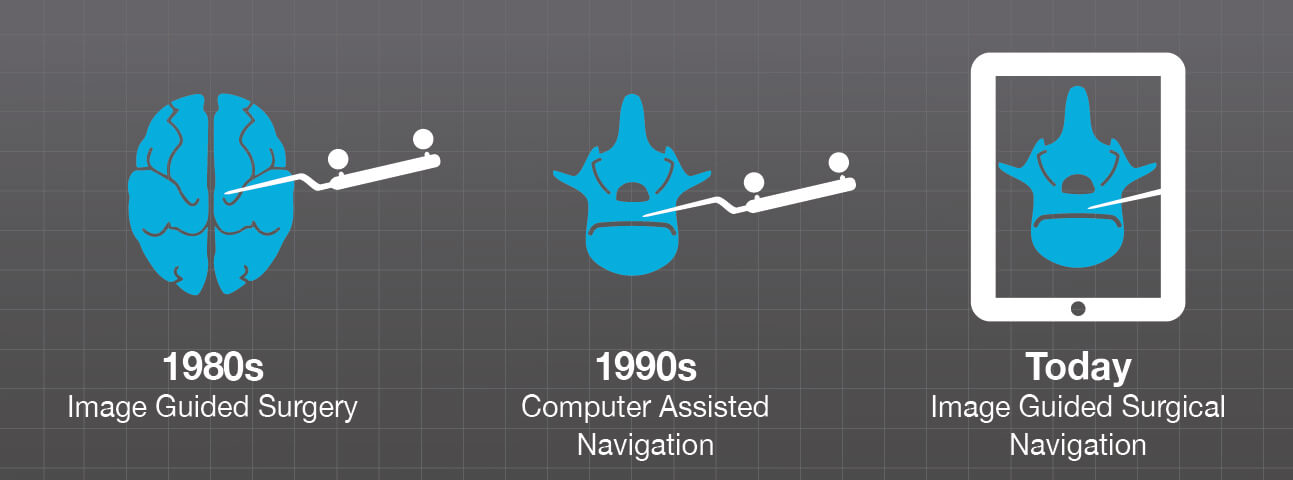
 Since the 1980s, surgeons have been using stereotactic image guidance intraoperatively for brain surgery to visualize patient anatomy, and monitor and correct procedural steps in real time. As these computer-based technologies evolved over time, their application has expanded to other surgical procedures.
Since the 1980s, surgeons have been using stereotactic image guidance intraoperatively for brain surgery to visualize patient anatomy, and monitor and correct procedural steps in real time. As these computer-based technologies evolved over time, their application has expanded to other surgical procedures.
In spine surgery, computer assisted navigation has been in use for around 30 years for pedicle screw implantation. In the last decade, spinal image guided surgery has greatly benefitted from important advancements like portable intraoperative computed tomography systems as well as high-speed, high-definition computerization and surgical tracking technology.1 Today, image guidance and intraoperative imaging are established and well-known components of many different surgical spine procedures.
While image guided surgery has been instrumental in the advancement of spinal procedures, the navigation system does not take the place of your doctor. These guidance systems enable your doctor to plan your spine procedure before surgery and help guide the surgical instruments used during surgery, enhancing overall accuracy.1 Surgical navigation is a technique to help place your spine implant or remove your tumor. It is not the implant itself; therefore, none of the instruments used during surgical navigation stay inside your body after surgery.
Is surgical navigation subject to FDA approval?
The Food and Drug Administration (FDA) is responsible for ensuring public safety by regulating medical devices. Surgical navigation systems are considered medical devices and therefore are subject to review and clearance by the FDA.